4-Week Stability Test: Your Fastest Route to Global Markets
An Expert Guide to the New European Regulations and Sustainability StandardsBringing a cosmetic product to market requires more than creativity – it requires scientific certainty about its safety and stability. Under EU Regulation (EC) No 1223/2009, every cosmetic product must remain safe and stable throughout its shelf life.
The first and most decisive step in this process is the 4-week accelerated stability test. At Annel, we combine laboratory precision with global regulatory expertise, helping brands make data-driven decisions before entering new markets.
We act as a single partner for your brand across three key regions: the European Union, the United Kingdom and the United States.
Why the 4-Week Test Provides Peace of Mind
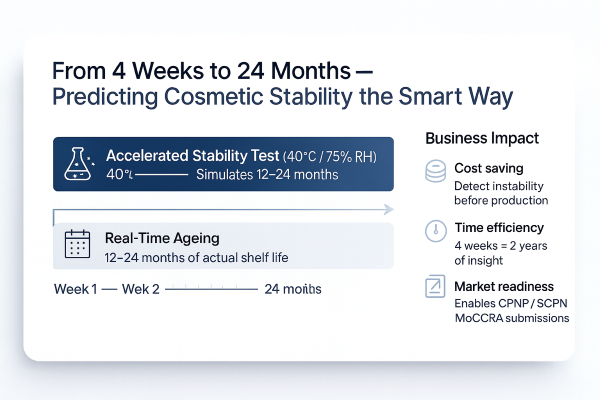
The 4-week accelerated stability test simulates 12–24 months of real-time ageing. It is the first analytical checkpoint determining whether a formulation is ready for long-term testing or requires costly reformulation. Four weeks in the laboratory can prevent two years of uncertainty and financial loss on the market.
Test parameters:- Organoleptic: colour, fragrance, texture.
- Physicochemical: pH, viscosity, emulsion stability.
- Packaging interaction: leakage, corrosion, mass loss.
The documentation from these tests becomes part of your Product Information File (PIF) required by EU law. A stable formulation is early scientific proof of durability – a key condition for responsible notification (CPNP / SCPN / FDA MoCRA).
From Analytical Rigour to Business Conclusions
At Annel, we apply rigorous analytical procedures derived from pharmaceutical stress-testing standards. This is not merely advanced technique – it is intelligent risk management for your brand.
- Reduced reformulation costs.
- Faster market readiness and global submissions.
- Forecasting of potential shelf-life limitations.
Our analysis identifies degradation points and chemical transformations that may threaten formulation integrity. We translate laboratory data into a clear business strategy.
Compliance: One Partner, Three Markets (EU RP / UK RP / US Agent)
No cosmetic product can be placed on the market in the EU, UK or USA without a designated Responsible Person (RP) or US Agent. The RP ensures that all product-safety data including the 4-week stability results are documented, verified and compliant with applicable law.
Annel acts as your Global Responsible Person, with offices in:
- 🇪🇺 Warsaw – EU Responsible Person (EU RP)
- 🇬🇧 London – UK Responsible Person (UK RP)
- 🇺🇸 Miami – US Agent under MoCRA
This is your key advantage: partnering with Annel ensures that your 4-week stability data are immediately integrated into a complete compliance system, including:
- CPNP / SCPN / FDA MoCRA notifications.
- Preparation and maintenance of the Product Information File (PIF).
- Post-market surveillance and regulatory reporting.
- Authorised Representative roles for CE and UKCA marking (for related products such as candles).
Frequently Asked Questions (FAQ)
What is a 4-week accelerated stability test?It is a short-term study that simulates up to two years of storage. By exposing samples to controlled stress conditions, it predicts how the product will behave under normal use.
Is the 4-week test mandatory?While not explicitly required, it forms part of the stability verification set out in Annex I of Regulation (EC) No 1223/2009. It is strongly recommended by the CTPA and FDA MoCRA as evidence of product safety.
How do the results affect market registration?Positive results allow progression to long-term testing and immediate preparation of the PIF. Instability requires reformulation before costly CPNP or SCPN submissions.
Ethical and Regulatory Assurance
Quality is a measurable and auditable process, not a slogan. Annel’s commitment to excellence is confirmed by membership in leading organisations such as the Cosmetic, Toiletry & Perfumery Association (CTPA) and the Polish Association of the Cosmetics and Detergent Industry (PACDI).
All procedures comply with Good Manufacturing Practice (GMP ISO 22716) and Annex I of Regulation 1223/2009. This ensures that every stability-test result we deliver serves as scientific evidence in regulatory audits and brand documentation.
Partnership with Annel: Turning Data into Market Confidence
Scientific testing and regulatory compliance are not obstacles – they are your fastest route to safe market success. Ready to verify your formulation’s stability and achieve global compliance?
Contact Annel today to receive a quote for our “Three Markets / One Partner” package.
References
- Regulation (EC) No 1223/2009 — European Parliament and Council on cosmetic products.
- Alsante K. M., Waterval J. (2003). A Stress Testing Benchmarking Study. Pharmaceutical Technology.
- Klick S., Muijselaar P. G. (2005). Stress Testing of Drug Substances and Drug Products. Pharmaceutical Technology.
- Kaloustian J. et al. (2002). Chemical, Chromatographic and Thermal Analysis of Rosemary (Rosmarinus officinalis). J. Appl. Polymer Sci., 83, 747–756.
- Baś W. (2020). Chemiczna analiza instrumentalna. AGH / WIMiC.
- Jakubiak I. (2021). Bezpieczny kosmetyk, czyli jaki? BMP Publishing.
- Gmelin E. (1999). Report of the Chairman of the Scientific Commissions and Working Groups. ICTAC News, 32/2.

