Importing Cosmetics into the EU: The Essential Guide for New Importers
Executive Summary
Importing cosmetics into the EU may appear straightforward, but the European Union enforces one of the strictest cosmetic regulatory frameworks in the world. New importers often underestimate the role of the Responsible Person (RP), the importance of CPNP registration, and the risks of inadequate documentation. This guide outlines the critical steps and common pitfalls that importers must understand to avoid customs delays, financial losses and compliance failures.
The Responsible Person (RP): Your Most Crucial Requirement
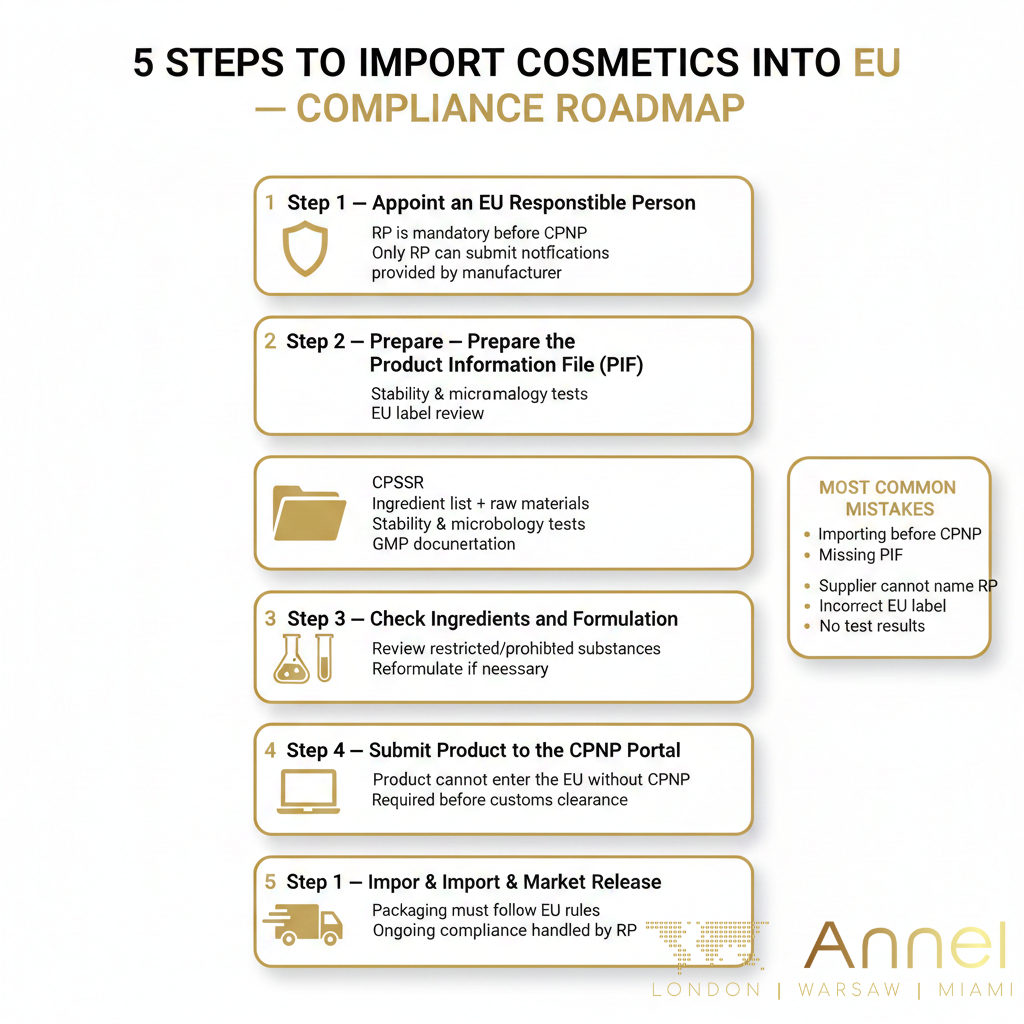
A cosmetic product cannot be notified on the CPNP without an appointed EU Responsible Person. Only the RP has permission to submit notifications via the Cosmetic Product Notification Portal (CPNP). If your supplier claims they already have a CPNP number but refuses to disclose the RP, this is a major red flag.
For full legal representation, see our EU Responsible Person service.
- Without an RP, the product cannot legally enter the EU market.
- A valid RP is required to submit or update product notifications.
- Lack of transparency typically indicates a non-compliant product.
The Hidden Risks of Importing Unregistered Cosmetics
Multiple cosmetic shipments are detained each month at EU borders due to incomplete documentation, missing CPNP notifications or safety concerns. Many of these cases appear in the EU Safety Gate (RAPEX).
- Shipments may be stopped or held indefinitely by customs.
- Importers may incur high storage fees.
- Documentation must be supplied before goods can be released.
- Non-compliant goods may be returned or destroyed.
What Documentation You Need for Compliance
To legally place cosmetics on the EU market, the Responsible Person must hold a complete Product Information File (PIF). All ingredients must also comply with the official EU CosIng ingredient database.
- CPSR – Cosmetic Product Safety Report
- Stability testing and compatibility
- Microbiological testing (challenge test where required)
- GMP documentation
- Ingredient specifications and raw material data
- EU-compliant labelling and warnings
More guidance on documentation can be found on our Cosmetic Regulatory Blog.
Questions You MUST Ask Your Supplier Before Ordering
- Is the product registered on the CPNP portal?
- Who is the appointed Responsible Person?
- What is the official CPNP number?
- Can the RP issue a mandate confirming your role as importer?
Working with New or Unregistered Cosmetic Brands
If the brand has not yet entered the EU, full compliance must be performed before import. This includes documentation collection, safety assessment, testing and CPNP notification.
- Direct coordination with the manufacturer
- PIF preparation and CPSR development
- Stability and microbiological testing
- CPNP submission
- Ongoing regulatory support via an approved RP
Download more resources for new brands via our Regulatory Guides.
Compliance Checklist for Importers
Conclusion
Importing cosmetics into the EU can be a profitable and scalable business strategy — provided compliance is taken seriously. Appointing a qualified Responsible Person, ensuring correct documentation and completing CPNP registration protects both your business and consumers. To discuss your project, visit our Contact Page.

