Even if you obtained a manual on cosmetic regulations — would you truly know everything, and for how long would that knowledge remain valid?
Why 2026 is a critical compliance year for cosmetic manufacturers
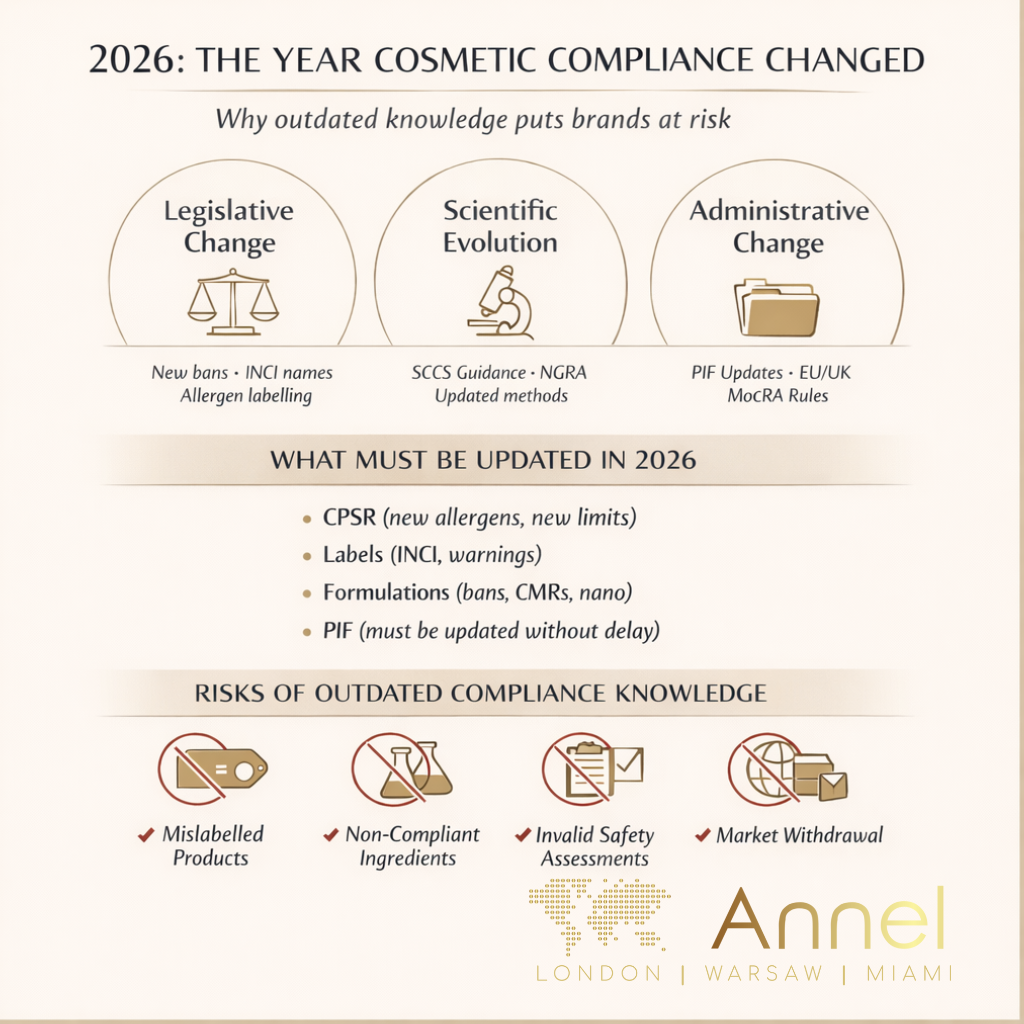
2026 marks one of the most significant regulatory years for cosmetic manufacturers operating across the EU, UK and US. Mandatory fragrance allergen labelling, updated INCI nomenclature, newly enforced ingredient restrictions, SCCS-driven changes and evolving post-Brexit and MoCRA obligations mean that regulatory knowledge acquired even a year ago may already be obsolete.
Compliance in cosmetics is not achieved through a manual, a one-off training session or historical assessments. It requires continuous scientific and regulatory alignment. Knowledge that was accurate at market entry can become legally invalid within months.
This article sets the regulatory baseline for 2026 and explains:
- why regulatory knowledge has a short operational lifespan,
- how ongoing changes affect compliance in the EU, UK and US,
- why manufacturers must view compliance as a continuous process rather than a one-time task.
Who this article applies to
- cosmetic manufacturers and brand owners operating in multiple regulatory markets,
- companies acting as, or working with, a Responsible Person (RP),
- regulatory teams responsible for maintaining lifecycle compliance,
- businesses seeking ongoing monitoring through resources such as the Cosmetic Regulatory Blog.
1. European Union: continuous regulatory change as a compliance constant
Regulation (EC) No 1223/2009 remains the foundation of EU cosmetic law and the most advanced safety framework worldwide. Its defining feature is continuous revision driven by scientific risk assessment — not regulatory stability.
1.1 The Annexes: the fastest-moving area of EU cosmetic law
The most frequent amendments affect:
- Annex II – prohibited substances
- Annex III – restricted substances
- Annex IV–VI – colourants, preservatives, UV filters
As a result, ingredient compliance knowledge becomes partially outdated within 3–6 months. A product that was compliant last year may now require reformulation, relabelling, market withdrawal or updated documentation.
1.2 EU regulatory changes impacting 2025–2026
CMR substances: Omnibus VII (Regulation (EU) 2025/877)
Over 20 new CMR bans apply from 1 September 2025.
Nanomaterials restrictions — Regulation (EU) 2024/858
Twelve nano-ingredients banned due to insufficient safety data.
February 2025: placing on the market prohibited
November 2025: making available prohibited
Ingredient restrictions — Regulation (EU) 2024/996
New limits for retinoids, arbutin, kojic acid, triclocarban and triclosan.
Fragrance allergen labelling — Regulation (EU) 2023/1545
Mandatory expansion from 26 to over 80 allergens.
Full compliance required by 31 July 2026.
Updated INCI nomenclature — Implementing Decision (EU) 2025/1175
New INCI names become mandatory from 30 July 2026.
Compliance implication (EU)
- CPSRs require scientific and regulatory review,
- labels must be updated under allergen and INCI changes,
- PIFs must be amended accordingly,
- some products may no longer be marketable without corrective action.
2. Scientific guidance: compliance extends beyond legislation
Safety assessments must reflect the latest SCCS guidance. The 12th SCCS Notes of Guidance introduced:
- New Approach Methodologies (NAMs),
- Next-Generation Risk Assessment (NGRA).
A CPSR based on outdated methodology may no longer be scientifically defensible. For scientific and regulatory alignment, see the Annel EU guidelines update.
3. United Kingdom: alignment without automatic synchronisation
The UK Cosmetics Regulation resembles the EU framework but evolves independently. Products sold in both markets require jurisdiction-specific compliance.
For clear definitions and regulatory scope, see the Annel regulatory explainer.
4. United States: MoCRA and regulatory uncertainty
Key 2025–2026 milestones include:
- Formaldehyde ban in hair straightening products (expected Dec 2025),
- Fragrance allergen disclosure (expected May 2026),
- Standardised talc testing (expected March 2026),
- GMP rule finalisation still pending.
Early MoCRA interpretations are already partially outdated. Updates available via FDA MoCRA guidance.
5. Why regulatory knowledge expires
Regulatory knowledge becomes outdated due to:
- legislative changes,
- scientific evolution,
- administrative and enforcement shifts.
The Product Information File must be updated whenever allergens, INCI names, safety data, formulation or packaging change. PIF retention is 10 years — but compliance is continuous, not historical.
Conclusion: 2026 requires continuous regulatory oversight
In cosmetic regulation, “static knowledge is a compliance risk”. Manuals, previous training and historical CPSRs provide a foundation — but not a guarantee of conformity.
In 2026, manufacturers must ensure:
- CPSRs are scientifically current,
- documentation reflects updated legal requirements,
- regulatory changes are monitored and implemented proactively,
- multi-market oversight is systematic, not reactive.
Compliance is no longer procedural — it is an operational responsibility.

