Annel Regulatory Compliance & Responsible Person services in the UK, EU & USA. Ensure safety, meet legal standards & expand globally with Annel!
Cosmetic products compliance consultants.
Cosmetics Regulatory
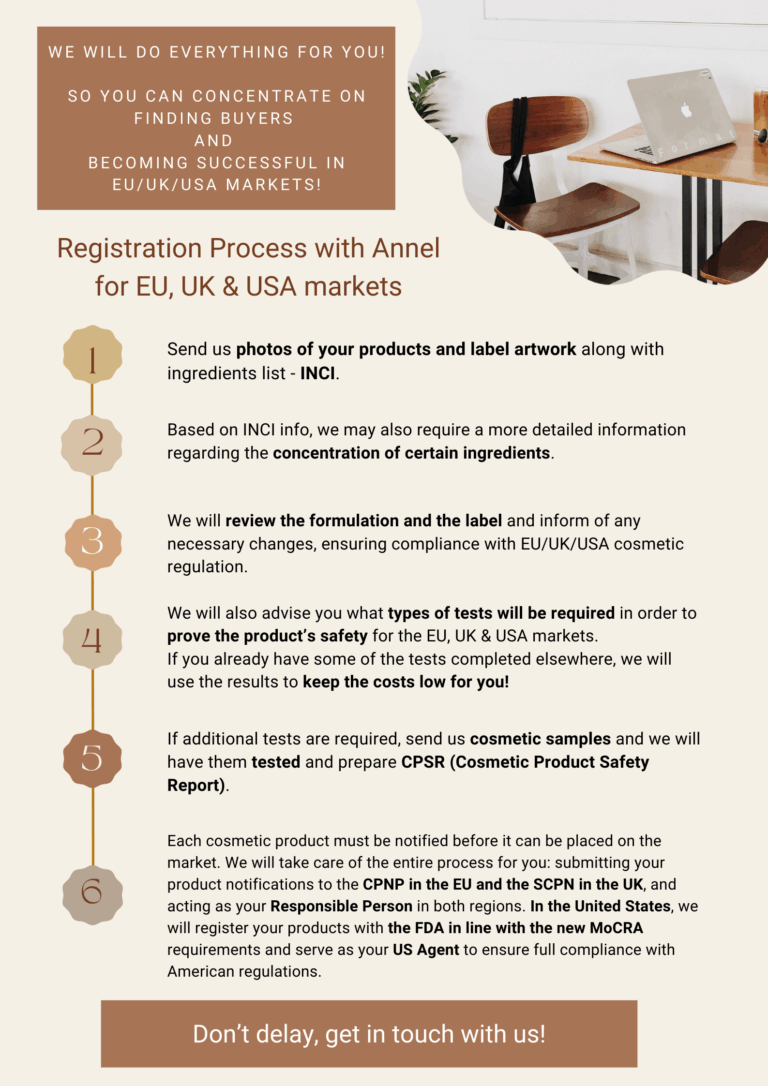
Registration Process with Annel
Ingredients Compliance with European Union and United Kingdom Regulations
REGULATION (EC) No 1223/2009 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 30 November 2009 on cosmetic products describes which substances and ingredients may be used in cosmetics sold in the EU and UK.
By having the INCI list of your product, we are able to confirm whether the ingredients in your cosmetic products are compliant with EU and UK regulations.
Cosmetics testing
According to EU Cosmetics Regulation 1223/2009, laboratories must test cosmetic products before they reach the European or UK markets. These tests follow officially approved procedures to ensure both product safety and regulatory compliance.
At Annel, we work with a wide range of laboratories, which allows us to offer our clients the best prices. We understand that time is money, and we work efficiently, maintaining high standards of accuracy in order to meet the deadlines set by clients.
A wide range of testing methods is accepted by UK and EU Authorities. These include:
Claim substantiation
Information on product labels, leaflets, and any other means of advertising must be supported by evidence.
All cosmetic products sold in the EU market must fulfill criteria for claims in terms of: legal compliance, truthfulness, evidential support, honesty, fairness, and informed decision-making.
At Annel, you can request experimental studies to be carried out to prove the efficacy of your products.
Microbiology testing
Cosmetic products placed on the EU market must meet strict safety criteria. Therefore, brands need to ensure their formulas are safe by confirming microbiological specifications for cosmetic products. At Annel, we offer microbiological testing through ISO-accredited laboratories, using methods approved by both EU and UK authorities.
We test for the following:
-
Total aerobic microbial count (ISO 21149:2017)
-
Yeast and mould count (ISO 16212:2017)
-
Pathogen testing, including:
-
Staphylococcus aureus (ISO 22718:2016)
-
Pseudomonas aeruginosa (ISO 22717:2016)
-
Candida albicans (ISO 18416:2015)
-
Escherichia coli (ISO 21150:2015)
-
Importantly, if the product passes these required microbiological tests, we then confirm its compliance with cosmetic safety standards.
Moreover, including microbiological testing results in your Product Information File (PIF) and Cosmetic Product Safety Report (CPSR) shows a professional commitment to consumer safety. It also ensures smooth product placement on the UK, EU, and U.S. markets.
Challenge test
Also known as the Preservative Efficacy Test (PET), the challenge test is required for the Product Information File as well as the Cosmetic Product Safety Report (CPSR). This test ensures that each finished product remains free from microorganisms that may be introduced during normal use. The test assesses whether the quality or safety of the product will be adversely affected during use (ISO 29621). At Annel, we conduct the challenge test according to ISO 11930:2019 standards. Your product will be exposed to five different strains of microorganisms, including:
Staphylococcus aureus
Pseudomonas aeruginosa
Candida albicans
Escherichia coli
Aspergillus brasiliensis
This procedure is conducted on days 7, 14, and 28 to evaluate the presence and survival levels of these microorganisms in the product.
Stability and compatibility
Stability testing is required to check the stability of a cosmetic product under various conditions, covering the period from manufacture to the product’s expiration. Cosmetic products must meet certain prescribed physical, chemical, and microbiological quality standards, ensuring functionality, safety, and aesthetics. Stability and compatibility tests evaluate whether the product maintains its quality standards over time during handling, transport, and storage under appropriate conditions. Based on the stability test results, we also assign the product’s shelf life and the period after opening. Packaging type affects product stability, as it protects the product from the external environment. Compatibility between packaging and product content is also tested to ensure no quality issues arise due to packaging materials.
Cosmetics stability test
Stability tests can be performed in real-time or through accelerated methods. The test duration and conditions may vary, as can the parameters monitored (e.g., pH, viscosity, density, and microbiological specifications). Accelerated stability tests generally involve keeping the product at elevated temperatures (37°C, 40°C, or 45°C) for a period of 3 months, or performing freeze-thaw tests. Additionally, testing may include exposure to sunlight if transparent packaging is used. Stability tests overseen by Annel meet ISO 18811:2018 standards.
Safety Assessment
A safety assessment is part of the Product Information File and the Cosmetic Product Safety Report (CPSR).
Our qualified EU safety assessors will conduct a safety assessment of your product in compliance with EU standards (1223/2009).
EU & UK Responsible Person for cosmetic products
Legal appointment required under Regulation (EC) 1223/2009 and UK SCPN.