Annel Regulatory Compliance & Responsible Person services in the UK, EU & USA. Ensure safety, meet legal standards & expand globally with Annel!
Cosmetic products compliance consultants.
News & Updates
PFAS in Cosmetics: What Changes in 2026 for UK, EU and USA Brands
PFAS, known as “forever chemicals”, are no longer only a sustainability topic. For cosmetic brands operating in the UK, EU and USA, PFAS is increasingly a practical compliance and business continuity risk.
The regulatory focus is shifting from individual substances to broader PFAS control. Brands are also seeing more scrutiny from market partners, including retailers and distributors, who expect evidence of control across formula, packaging, and supply chain.

Where PFAS Risk Concentrates in Cosmetics
PFAS related risk is typically highest in performance driven categories, where durability, water resistance and transfer resistance are core product outcomes.
- long wear foundations and base products
- waterproof and smudge resistant eyeliners and mascaras
- transfer resistant colour cosmetics built on strong film formation
PFAS questions can also arise even when the formula looks clean, due to indirect sources such as raw material contamination, contact with process materials, or packaging components.
UK and EU: Broader Scope and Earlier Action
In the UK and EU, PFAS is increasingly treated as a high risk chemical group. The operational impact is straightforward: a launch day compliance check is not enough. Brands need horizon monitoring and the ability to act early through reformulation, supplier changes, and documentation updates.
USA: Uneven Requirements and One Defensible Standard
In the USA, PFAS expectations can vary by jurisdiction and by retailer policy. This creates inconsistent definitions and uneven enforcement signals. The most defensible route is one internal PFAS standard applied across the full portfolio, supported by supplier requirements and documented quality controls.
The Substitution Trap: Why a Simple Swap Can Fail
Replacing PFAS with other fluorinated alternatives or short chain PFAS can create a substitution trap. The risk is not removed, it shifts to a different substance profile and can become harder to control and defend. A structured PFAS strategy is usually more robust than a one to one replacement decision.
What Brands Should Do Now
- define what PFAS free means for your business, including scope and acceptance criteria
- prioritise high risk product lines first, especially long wear and waterproof ranges
- set supplier requirements, including declarations and change notification obligations
- review risk beyond the formula, including process materials and packaging
- maintain a clear evidence trail of decisions and controls for UK, EU and USA
Cosmetics regulation news and updates
Latest insights and updates in cosmetics regulation for EU/UK brands. Key regulatory changes, ingredient restrictions, compliance requirements, and industry news.
Cosmetics regulation news and updates
Stay up to date with the latest developments in cosmetics regulation across the EU and UK. This overview page collects regulatory updates, industry changes, and compliance insights relevant to manufacturers, importers, distributors, and Responsible Persons.
Here you will find ongoing updates on cosmetics regulation including ingredient restrictions, SCCS opinions, labelling requirements, PAO and durability rules, safety assessment changes, and enforcement trends affecting the European and British markets.
This introduction improves search visibility by helping search engines understand that this page provides authoritative coverage of cosmetics regulation topics.
England Confirms Ban on Wet Wipes Containing Plastic from May 2027

On 18 November 2025, the UK Government formally adopted the Environmental Protection (Wet Wipes Containing Plastic) (England) Regulations 2025. The legislation will come into force on 19 May 2027, introducing a complete ban on selling or supplying wet wipes containing plastic to consumers in England.
Under the regulation, a “wet wipe” is defined as a pre-wetted, single-use non-woven fabric, a category which also includes pre-wetted sheet masks and face masks.
The purpose of the ban is to reduce plastic pollution and limit microplastics entering rivers, coastal waters and sewage systems.
Exemptions
- medical use under the supervision of healthcare professionals
- non-promoted sales via registered pharmacies (including online)
- business-to-business and local authority supply
Wales and Northern Ireland have already implemented similar legislation, and Scotland is expected to introduce equivalent measures in due course.
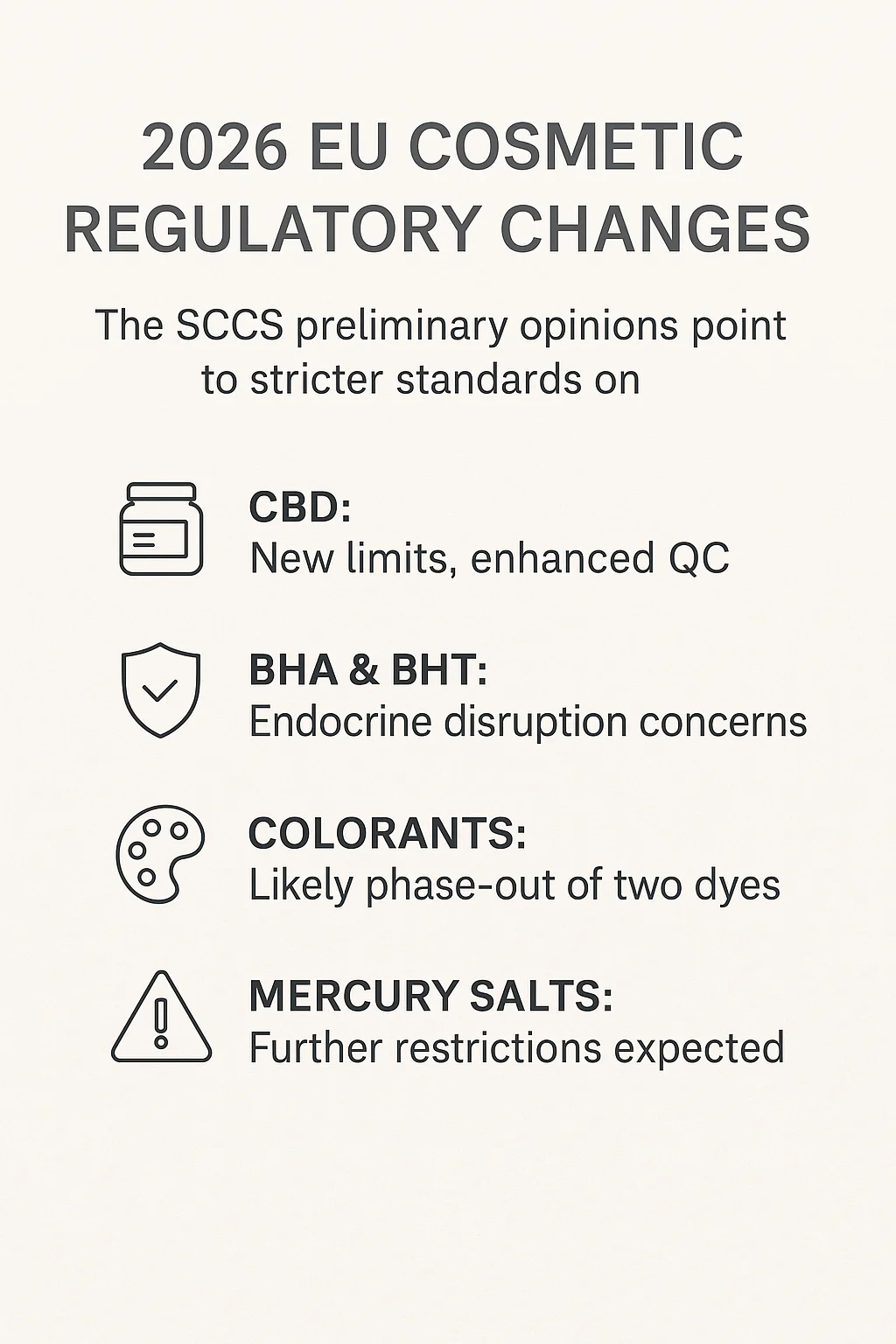
REGULATORY COMPENDIUM 2026: Key Takeaways from the Latest SCCS Preliminary Opinions
From January 2026, the EU cosmetics industry will face one of the most impactful regulatory updates in recent years. The latest preliminary opinions issued by the Scientific Committee on Consumer Safety (SCCS) cover CBD, antioxidants (BHA/BHT), and several colourants with potential genotoxic concerns.
For companies operating in the EU market, this signals the need for immediate formula review and supply chain verification.
SCCS Public Consultation Deadlines (January 2026)
- BHA, Basic Brown 16, Basic Blue 99 — Opinions 1682/25, 1684/25, 1683/25 — Deadline: 19 January 2026
- CBD, Thiomersal, Phenylmercuric salts — Opinions 1685/25, 1686/25 — Deadline: 21 January 2026
Takeaway:
The short consultation period suggests rapid amendments to Regulation (EC) No 1223/2009.
CBD – New Safety Limits and Control Requirements
The SCCS preliminary opinion concludes that CBD can be considered safe only under strict concentration controls:
- Maximum concentration: up to 0.19% (dermal & oral care)
- Maximum acceptable THC impurity: 0.00025%
- Excludes inhalable/pulmonary exposure formats
Business impact:
- strengthened quality control and THC quantification
- supplier reassessment
- updated safety assessments and PIF documentation
BHA & BHT – Antioxidants under Endocrine Disruption Review
BHA (Butylated Hydroxyanisole)
- safe only up to 0.07% in dermal products
- evaluated with caution due to endocrine-disrupting potential
BHT (Butylated Hydroxytoluene)
- 0.001% – mouthwashes
- 0.1% – toothpastes
- 0.8% – all other cosmetics
Relevance: companies should prepare for formula adjustments and strengthened toxicological justification.
Genotoxicity Concerns: Colourants Likely to Be Phased Out
Basic Brown 16
- evidence of mutagenic and genotoxic potential
- conclusion: not safe for use
Basic Blue 99
- highly variable chemical composition
- multiple impurities and isomers
- unsafe due to uncertainty
Regulatory expectation: likely full phase-out; immediate reformulation recommended.
Mercury Salts (Thiomersal & Phenylmercuric Salts)
SCCS is currently evaluating these preservatives. Given the toxicological profile of mercury compounds, further restrictions or a full ban appear highly probable.
Operational Impact Across the Supply Chain
Manufacturers
- Reformulate CBD, BHA, colourant-containing products
- Strengthen raw material qualification
- Update toxicological profiles & PIF documentation
Importers
- Urgent compliance audits of non-EU formulations
- Inventory strategies for at-risk ingredients
Exporters
- Align EU formulas with global lines or adopt EU as baseline
- Mitigate customs & distribution delays linked to restricted substances
Strategic Conclusions
- EU moving toward stricter thresholds for endocrine/genotoxic substances
- Transition periods may be short or absent
- January 2026 = operational deadline for audits
- Proactive compliance reduces withdrawal & reformulation risks
Summary
This compendium outlines the most critical regulatory updates expected following the SCCS preliminary opinions. For cosmetic companies, 2026 will require:
- coordinated reformulation,
- supply chain revalidation,
- strong regulatory alignment across markets.
Proactive compliance remains the most reliable approach to ensuring business continuity and market access.
Final SCCS Opinion on Tea Tree Oil: Safety Limits and Regulatory Impact
The Scientific Committee on Consumer Safety (SCCS) has published its final opinion on Tea Tree Oil (TTO) following a proposal to classify it as a reproductive toxicant (Repr. 1B) under the EU CLP Regulation.
Official SCCS opinion:
https://health.ec.europa.eu/publications/sccs-opinions_en

Proposed Repr. 1B Classification by ECHA
The ECHA Risk Assessment Committee (RAC) recommended classifying TTO as Repr. 1B. This classification would normally trigger an automatic ban in cosmetics unless a derogation under Article 15(2)(d) of Regulation 1223/2009 is granted.
ECHA RAC opinions:
https://echa.europa.eu/opinions-of-the-committee-for-risk-assessment-on-harmonised-classification-and-labelling
SCCS Final Opinion: Safe Use at Defined Levels
The SCCS considers TTO safe for adult cosmetic use at the following maximum concentrations:
- 2.0% in shampoos
- 1.0% in wash-off gels
- 0.1% in face creams
The opinion accounts for aggregated exposure and the potential Repr. 1B classification.
Stability, Oxidation and ISO 4730
Safe use depends heavily on avoiding oxidation. Key points:
- oxidised TTO significantly increases the risk of skin sensitisation
- use of antioxidants and protective packaging is recommended
- ISO 4730 specifications must be met
Spray and aerosol products are excluded from safe use due to inhalation risks.
ISO link:
https://www.iso.org/standard/72198.html
Divergence Between EU and UK
The UK Health and Safety Executive (HSE) does not support classifying TTO as Repr. 2 under the UK CLP framework. This results in a regulatory divergence between the EU and UK markets.
UK CLP resources:
https://www.hse.gov.uk/chemical-classification/index.htm
Key Implications for Industry
- adjust formulas to SCCS safe limits
- update stability protocols
- ensure compliance with ISO 4730
- re-evaluate aerosol TTO products due to inhalation risks
EU Cosmetics Regulation Update – Effective 1 November 2025
The European cosmetics sector is preparing for a significant regulatory shift. From 1 November 2025, three new Commission Regulations — (EU) 2024/996, (EU) 2024/858, and (EU) 2025/877 — introduce extensive amendments to the EU Cosmetics Regulation (EC No 1223/2009).
These acts impose:
- New concentration limits and warnings for Vitamin A (retinoids)
- Restrictions for endocrine disruptors such as Alpha-Arbutin, Kojic Acid, Genistein
- Bans on specific nanomaterials and CMR substances
- Complete withdrawal of Triclosan, Triclocarban and UV filter 4-MBC
This marks the most comprehensive regulatory revision since 2013, aiming to strengthen consumer safety and product transparency.
Our Regulatory Affairs Division has published a complete briefing — “EU Cosmetics Compliance Brief 2025” — summarising every deadline, limit, and scientific reference in one document.
*Ensure your cosmetic formulations and documentation are compliant ahead of the November 2025 deadlines.*
Regulatory Update – 64 CMR Substances Prohibited in UK Cosmetics

Breaking News for the Cosmetics Industry!
The UK Office for Product Safety and Standards (OPSS) has added 64 substances classified as Carcinogenic, Mutagenic, or Reprotoxic (CMR) to Annex II of the UK Cosmetics Regulation.
Key Details:
These substances are now prohibited from being used in cosmetic products. Examples of newly banned substances include:
- Methylene di-t-butylcresol (CMR 1B) – Entry 1681
- Methyl isobutyl ketone (MIBK) – Entry 1691
- Benzophenone – Entry 1704
- Trimethylolpropane triacrylate – Entry 1712
- Margosa extract – Entry 1722
🗓 Compliance Deadlines
For entries 1680–1730:
Placing on market: 20 April 2025
Off-shelf (making available): 20 October 2025
For entries 1731–1743:
Placing on market: 2 September 2025
Off-shelf (making available): 2 March 2026
How Does It Compare to EU Regulations?
The EU has already banned these substances under earlier deadlines. UK deadlines allow for more transition time, but dual compliance is necessary for businesses operating in both regions.
Act Now to Stay Compliant!
- Review product formulations.
- Audit supply chains and update documentation.
Need Help?
Contact us for guidance on reformulation and compliance strategies tailored to your needs.
New Guidelines for Online and Distance Sales of Cosmetic Products Under GPSR Article 19
Starting December 13, 2024, the General Product Safety Regulation (GPSR) will be directly applicable in all EU Member States, introducing new information requirements for online sales. While cosmetic products are primarily regulated by the Cosmetic Products Regulation (CPR), GPSR Article 19 complements these rules specifically for distance sales.
What’s changing?
Manufacturer or Responsible Person details: name, postal address, and electronic address.
Product identification: a picture, product type, and other identifiers.
Warnings and safety information: must be presented in a language understood by consumers.
Who is responsible? Responsibility is shared between:
The Responsible Person (under CPR): ensures the information is accurate and up-to-date.
Online retailer: must ensure the information is clearly visible on the sales platform.
Learn more: For detailed guidance and interpretation of these requirements, refer to the attached document. Make sure your online offers comply with the new rules.
Microplastics EU & UK Requirements (09/2023)
Microplastics pose one of the most serious threats to our planet. Introduced into cosmetics to enhance their texture and effectiveness, these microscopic plastic particles can significantly harm aquatic environments and human health. In response, the European Union and the United Kingdom are implementing new regulations aimed at reducing the presence of microplastics in cosmetic products.
Why Are Microplastics a Problem?
Microplastics are plastic particles under 5 mm, originating from the breakdown of larger plastics or from cosmetic products like exfoliants, shampoos, and creams. Their small size allows them to easily enter aquatic environments, accumulate in organisms, disrupt ecosystems, and eventually enter the human food chain.
New Regulations and Their Purpose
The EU and UK have introduced stricter rules to reduce microplastic pollution. Key measures include:
- Ban on Microplastics: Products such as exfoliants and facial cleansers may no longer contain microplastics.
- Limiting Soluble Microplastics: If microplastics are necessary, they must be biodegradable and formulated to reduce long-term pollution.
Why Download Our Brochure?
- Specific products where microplastics are banned
- Local microplastics requirements in EU Member States
- Key terminology of the regulation
- Microplastics criteria and possible derogations
- Case studies with explanations
- FAQ and typical uncertainties
- Guidelines for “microplastic-free” claims
Our daily choices impact the environment — learn the regulations and help reduce microplastic pollution together.
Download and share our free brochure now to discover how new microplastics regulations will impact your products in the EU and the United Kingdom. Let’s be conscious consumers and work together to protect our planet.
Unlock the Ultimate Guide for Brand Owners: Navigating the Current Plastic Tax in the UK!
Are you a brand owner or a business leader looking to stay ahead of the curve when it comes to the latest plastic tax regulations in the UK? Plastic waste is a pressing issue, and understanding how it affects your business is crucial. Introducing our comprehensive brochure designed exclusively for you: "Demystifying the Plastic Packaging Tax."
In today's ever-evolving business landscape, staying informed is the key to success. The Plastic Packaging Tax (PPT), introduced in the UK on April 1st, 2022, has significant implications for brand owners. It's not just another tax; it's a transformational step towards a more sustainable future.
The PPT promotes sustainability and encourages a circular plastic economy. As a brand owner, you have a unique opportunity to lead by example and make a positive impact on the environment.
What's Inside Our Guide?
- Key Definitions and Terms: Understand the essential concepts behind PPT.
- Companies Subject to PPT: Learn requirements and compliance obligations.
- Charge Rate: Discover how the tax rate is calculated.
- Types of Packaging: Identify which materials fall under PPT.
- Case Studies: Explore real-world examples of reducing plastic use.
This brochure isn’t just about compliance; it’s about seizing the opportunity to lead the transition toward a cleaner, more sustainable future.
As a brand owner, your actions matter.
By understanding the ins and outs of the Plastic Packaging Tax, you can reduce your environmental impact and reinforce your brand as an eco-conscious leader.
Knowledge is power. Download our free brochure now and become a trailblazer in sustainable branding. This is your chance to take the lead, set new standards, and make a lasting positive impact on our planet.
*Download and share our free brochure now and pave the way for a plastic-free, sustainable future for your brand!*
Labelling Requirements for Responsible Person (RP) in the UK Cosmetics Market

Following the UK's departure from the EU Single Market in December 2020, the UK Cosmetics Regulation came into effect. This regulation included a two-year transitional provision, allowing companies to make the necessary changes, specifically addressing labelling compliance for cosmetics.
The original two-year grace period was initially set to conclude on 31 December 2022. After this date, all products placed on the GB market would have been required to fully comply with the UK Cosmetic Regulation, or alternatively, be withdrawn from the market.
The Office for Product Safety and Standards (OPSS) has reviewed the requirement to label products with the UK Responsible Person and has decided to extend this transitional provision. This extension now covers a total period of five years, effectively until 31 December 2025.
This means that until this extended date, compliance with Article 19(1)(a) of the EU Cosmetic Products Regulation (EU CPR) will satisfy the name and address of the Responsible Person and country of origin requirements.
This consideration does not apply to non-EU regulations. Additionally, companies must still comply with all other UK Cosmetics Regulation requirements, including notification and ingredient compliance.
The extension provides businesses with additional time to update their product labels. We encourage all companies to use this period wisely and ensure their products align with the updated guidelines.
CLP Legislation – Classification, Labelling and Packaging

CLP legislation controls how and what you must communicate about your products. For candle makers, the most important element of CLP is label design.
Brexit’s Impact on Candle Making
EU CLP – European Union | GB CLP – United Kingdom
Brexit did not significantly impact most aspects of candle making. CLP legislation in the UK has only minor differences compared to EU CLP. However, if you want to sell candles in both the EU and the UK, you must comply with both EU CLP and GB CLP.
CLP-Compliant Label Must Include:
- Supplier identification (candle maker)
- Weight or mass of the candle
- Product name or identifier
- Hazard pictograms
- Signal word
- Hazard statements
- Precautionary statements
- Relevant supplementary information
- Unique formula identifier (UFI)
Labels must be visible when the product is at rest in its normal position.
The label must be in the language of the country where the candle will be sold.
Additionally, the label must include:
Name, address and phone number of the candle maker or authorised representative,
nominal quantity (weight of jar, wax, lid), product name, and all substances classified as:
acute toxicity, skin corrosion, serious eye damage, germ cell mutagenicity,
carcinogenicity, reproductive toxicity, respiratory or skin sensitisation,
specific target organ toxicity (STOT), aspiration hazard.
The Safety Data Sheet (SDS) contains hazard pictograms, signal words (e.g. WARNING), hazard statements, precautionary statements and supplementary information — all of which must be included on the label.
UFI – Unique Formula Identifier
The UFI is a 16-digit code used by Poison Centres to quickly identify the contents of your candle in an emergency.
UFIs are not required for products sold exclusively in the UK.
You can generate your UFI using the online tool provided by the European Chemicals Agency (ECHA).
For more information, please consult the CLP documents .

50th IFRA Standard Amendment
We would like to remind you of the 50th IFRA Standards Amendment. The document assumes withdrawal of Mintlactone from the list of fragrance ingredients.
The 50th amendment is effective from 30 August 2021 for newly introduced products, and from 30 August 2022 for products already on the market. The document directly concerns suppliers of raw materials; however, cosmetic manufacturers should contact their suppliers and verify whether the new guidelines require changes to existing product formulations.
These changes should be of particular interest to manufacturers whose formulations contain Mintlactone.
For more information, please consult the IFRA documents .
We support others

We are happy to inform you that Annel is supporting No Shame Foundation , which helps and educates people who suffer from psoriasis and other skin diseases.
No Shame Foundation is currently developing a cosmetic ranking on their website, presenting information about cosmetic ingredients and the effectiveness of products formulated for people suffering from various skin conditions.
This could be the beginning of a cooperation between our clients and the No Shame Foundation, as many of our customers may be interested in presenting their products to consumers in Poland. The Foundation is also planning to publish their website in English to reach a wider audience.
To see the cosmetic ranking, please click here: No Shame Foundation – Rank
The use of Butylphenyl Methylpropional shall be prohibited in cosmetic products from 1 March 2022.

EU bans the use of a common fragrance ingredient: Butylphenyl Methylpropional (also known as BMHCA or Lilial).
European Commission Delegated Regulation (EU) 2020/1182 — the 15th Adaptation to Technical Progress (ATP) of the CLP Regulation — was published in the Official Journal of the European Union on 11 August 2020.
The fragrance ingredient Butylphenyl Methylpropional (Lilial, BMHCA) was classified as toxic to reproduction (Repr. 1B – CMR 1B).
By default, substances classified as CMR are banned under the European Cosmetics Regulation. Consequently, Butylphenyl Methylpropional will be prohibited from cosmetic products from 1 March 2022 and added to Annex II.
Exemption conditions for CMR category 1A and 1B substances include:
- Compliance with the food safety requirements in Regulation (EC) No 178/2002.
- No suitable alternative substances available.
- The application is for a specific product category with known exposure.
- The substance has been evaluated as safe by the SCCS.
BMHCA does not meet the criteria for exemption. It will be added to Annex II from 1 March 2022.
Products containing this ingredient must be off the shelf by this date.
Reformulation is required, including a new CPSR and potentially updated labelling.
49th IFRA Standard Amendment

We would like to remind you of the 49th IFRA Standards Amendment. The document introduces changes within the improved methodology for quantitative risk assessment, leading to a re-establishment of product classes.
Additional standards have been introduced, including new restrictions and the withdrawal of certain fragrances. Restrictions on the use of phototoxic substances in rinse-out products have also been included.
The 49th amendment is effective from 10 February 2021 for newly introduced products, and from 10 February 2022 for products already on the market.
Although the document directly concerns raw material suppliers, cosmetic manufacturers should contact their suppliers and verify whether the new guidelines will require changes to existing product formulations.
These changes should be of particular interest to manufacturers whose formulations contain citrus essential oils.
For more information, please consult the official IFRA documentation .
